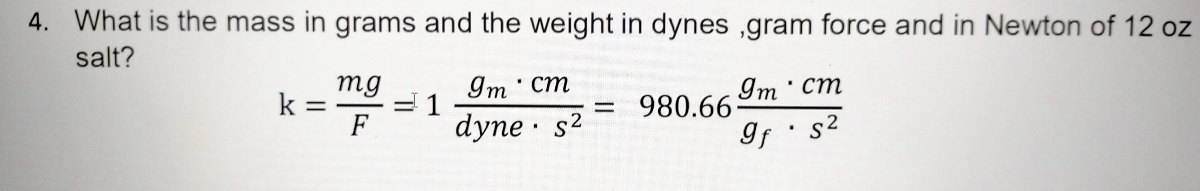A force of 72 dyne is inclined to the horizontal at an angle of 60°. Find the acceleration in a mass of 9 g, which moves in a horizontal direction. - Find 4
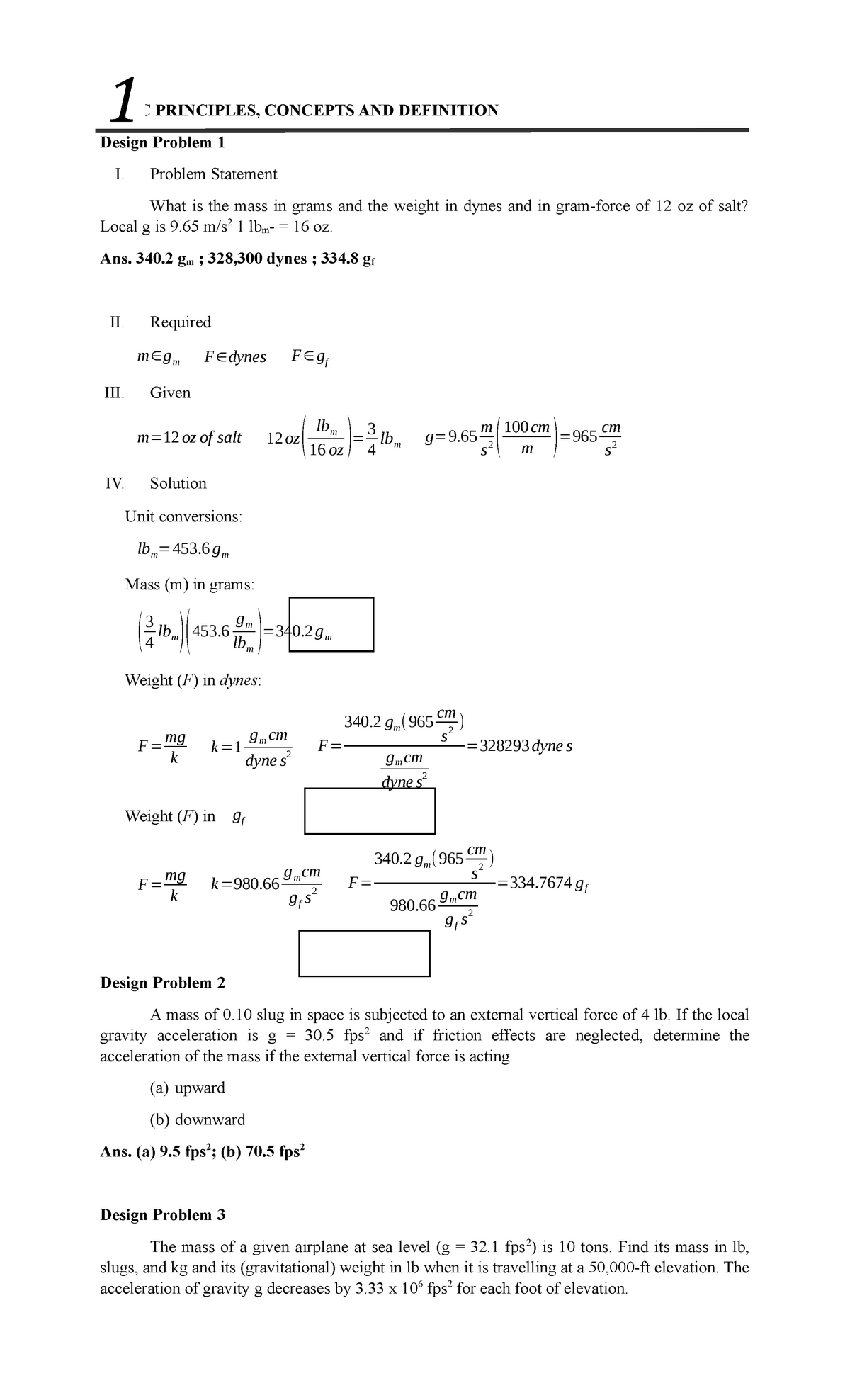
Prob-set-1 - Prob set - BASIC PRINCIPLES, CONCEPTS AND DEFINITION Design Problem 1 I. Problem - Studocu

SOLVED:If at a pressure of 10^6 dyne / cm^2, one gram mole of nitrogen occupies 2 ×10^4 c.c. volume, then the average energy of a nitrogen molecules in erg is : (Given:

Question Video: Finding the Rate of Change of the Mass of a Ball as It Moves through a Dusty Medium | Nagwa